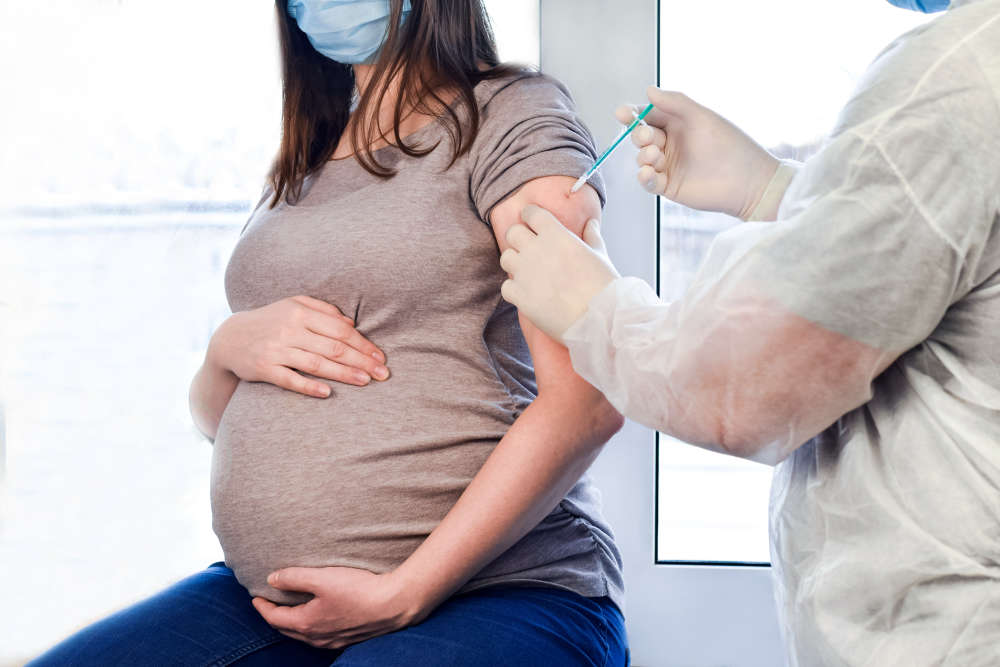
A national COVID-19 vaccine study is calling for more pregnant women from across the south to help discover the most effective use of booster vaccines during pregnancy.
The study is seeking low-risk, pregnant women aged 18 to 45 who are carrying a single baby and are between 13 to 36 weeks gestation.
Running at University Hospital Southampton (UHS), the Preg-CoV study currently has around 300 pregnant women taking part nationally and aims to determine the best vaccine schedules and doses to protect women and their babies against COVID-19.
The study compares vaccines currently being used for the UK vaccination programme (Pfizer/BioNTech and Moderna), as well as new vaccines as they are approved for use such as Novavax.
The research is also assessing the immune response to different doses and intervals between vaccines during pregnancy and participants may also be eligible to choose to receive their COVID-19 booster dose alongside the routine whooping cough vaccine.
Current UK guidance is that the COVID-19 vaccination should be offered to pregnant women at the same time as the rest of the population.
Dr Chrissie Jones, Southampton study lead and honorary consultant in paediatric infectious diseases and immunology at UHS, said:
“The COVID-19 vaccination in pregnancy has been proven to be safe in hundreds of thousands of women and protects them against severe infection.
“Now we need to understand the very best ways to protect women and their infants with booster doses and so are seeking more pregnant women from across the south to take part.
“This study will give us important information about the spacing of vaccines in pregnancy and whether a half dose of the booster is as good as the full dose of booster."
The study launched in August 2021, and will involve around 700 pregnant women in total at sites across England. It is supported by the National Institute for Health and Care Research (NIHR).
Pregnant women in the study will receive monitoring and additional support through study visits compared to those who receive their vaccine outside of the study.
They will also be provided with access to a 24-hour phone line should they have any questions for the trial team and an electronic diary to record any symptoms. They will also be reimbursed for travel to their study appointments.
Professor Paul Heath, chief investigator and professor of paediatric infectious diseases at St George’s, University of London, said:
“We are delighted that around 300 pregnant women have joined the study so far, but we need even more pregnant women across the country to participate.
“Not only will they receive special monitoring and support around their vaccines, but they will help shape future guidelines and protect pregnant women in the future. It is a really worthwhile, helpful thing to do.”
All participants and their babies will be followed up until one year after delivery.
If you are interested in the study, or know someone who could be eligible, you can find out more by visiting the study’s website .

 Engine Problems Lead To Yarmouth RNLI Callout To Osborne Bay
Engine Problems Lead To Yarmouth RNLI Callout To Osborne Bay
 Ryde Academy Students Donate To Two Worthy Causes At Christmas
Ryde Academy Students Donate To Two Worthy Causes At Christmas
 Road Improvement Works To Get Underway From The Very Start Of The New Year
Road Improvement Works To Get Underway From The Very Start Of The New Year
 Isle of Wight Youth Trust Benefits From Big Give Support
Isle of Wight Youth Trust Benefits From Big Give Support
 Council Chair 'Furious' Over Floating Bridge Replacement Fiasco
Council Chair 'Furious' Over Floating Bridge Replacement Fiasco
 Fire Service Travel To Island Contributes To Huge Budget Overspend
Fire Service Travel To Island Contributes To Huge Budget Overspend
 Department Approaching Completion In Ryde As Opening Date Announced
Department Approaching Completion In Ryde As Opening Date Announced
 Guidance Issued Over Sharps Disposal As Waste Teams At Risk
Guidance Issued Over Sharps Disposal As Waste Teams At Risk
 Police Warnings Over E-Scooters Ahead Of Christmas Day
Police Warnings Over E-Scooters Ahead Of Christmas Day
 Controversial Freshwater Housing Proposal Turned Down
Controversial Freshwater Housing Proposal Turned Down
 Rare Mantis Shrimp Discovery Made In Waters Off Isle Of Wight
Rare Mantis Shrimp Discovery Made In Waters Off Isle Of Wight
 Man In 50s Confirmed Dead Following Newport Crash
Man In 50s Confirmed Dead Following Newport Crash
 Pedestrian In Hospital With Serious Injuries Following Early Morning Motorcycle Crash
Pedestrian In Hospital With Serious Injuries Following Early Morning Motorcycle Crash
 Cowes Stately Home To Have Facilities Upgraded As Part Of Approved Renovation
Cowes Stately Home To Have Facilities Upgraded As Part Of Approved Renovation
 Ofsted Deliver Glowing Report For 'Welcoming' And 'Highly Ambitious' Island Primary School
Ofsted Deliver Glowing Report For 'Welcoming' And 'Highly Ambitious' Island Primary School
 Help Available For Islanders To Cut Energy Bills
Help Available For Islanders To Cut Energy Bills
 Cowes Lifeboat Performs Mid-Solent Rescue Of Fishing Boat
Cowes Lifeboat Performs Mid-Solent Rescue Of Fishing Boat
 Christmas Shopping With A Difference In Ryde – Half A Mile Out To Sea!
Christmas Shopping With A Difference In Ryde – Half A Mile Out To Sea!
 Home-From-Home' Nursery Given 'Outstanding' Ofsted Grade
Home-From-Home' Nursery Given 'Outstanding' Ofsted Grade


